Decision Autonomy in Pragmatic Clinical Trials

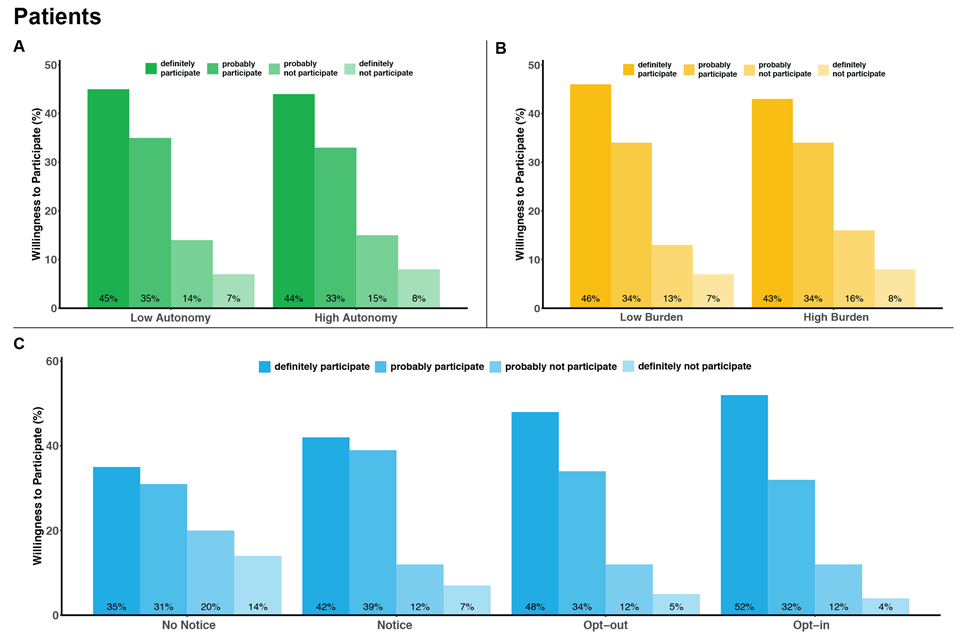
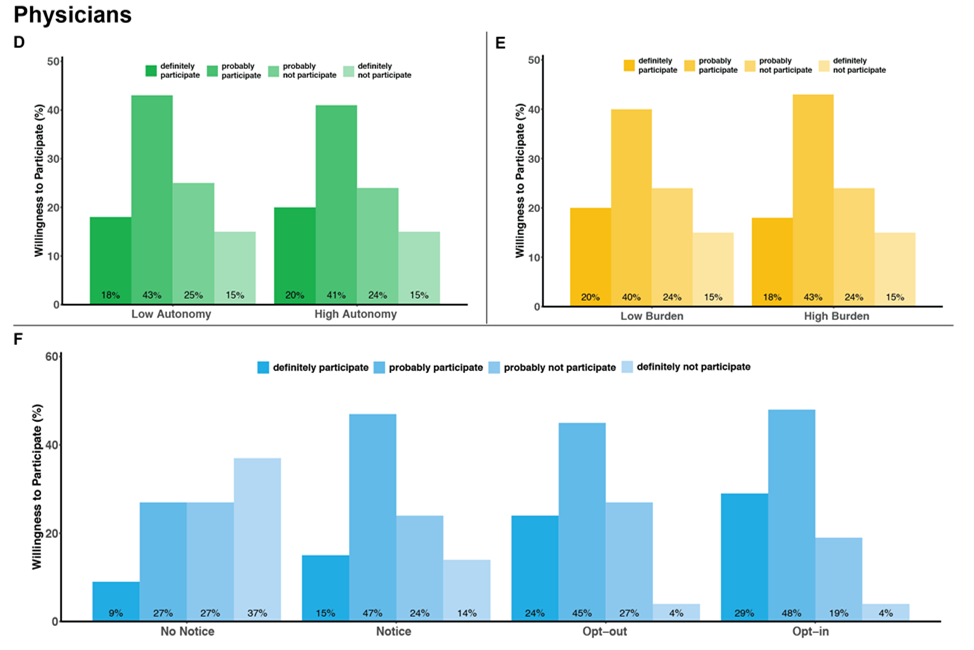
The "learning health care system" - in which generalizable knowledge is produced in concert with the delivery of clinical care - is increasingly recognized as having tremendous potential for improving health care quality and reducing costs. Pragmatic trials embedded within usual clinical care delivery are designed to maximize the external validity of the knowledge they generate by comparing the effectiveness, benefits, and harms of interventions under real-world conditions. However, pragmatic trials often have design features that raise ethical concerns regarding the loss of patient and physician autonomy that they may impose, especially if entire hospitals or clinics are randomized to a specific treatment approach. As promising as pragmatic trials may be for improving the quality and costs of care, little empirical work has been done to address these ethical concerns. Our mixed-methods study was conducted in two phases. In Phase 1, we undertook semi-structured interviews with 32 patients with end-stage renal disease (ESRD) receiving maintenance hemodialysis and 24 nephrologists to understand how they each value physician decisional autonomy and informed consent in a hypothetical standard-of-care trial of hemodialysis session duration compared with a clinical care protocol. In Phase 2, we quantified how strongly patients and physicians value physicians' treatment autonomy relative to other desirable clinical trial features such as minimization of participant burden and opportunity for informed consent. We conducted a survey-based discrete choice study among 200 patients with ESRD and with 203 nephrologists to determine the relative importance of these study attributes on willingness to participate in a hypothetical pragmatic clinical trial comparing two approaches to blood pressure management for patients receiving maintenance hemodialysis.
Sponsors
-
National Institute for Diabetes and Digestive and Kidney Diseases